Abstract
Background. Adverse events are relatively more common in the follow-up of primary myelofibrosis (PMI) than in other Ph- myeloproliferative neoplasms, with higher morbidity and mortality rates, as reported in several retrospective studies. At present, however, prospective data on the early incidence (<4 years from diagnosis) of these complications in PMI are not available.
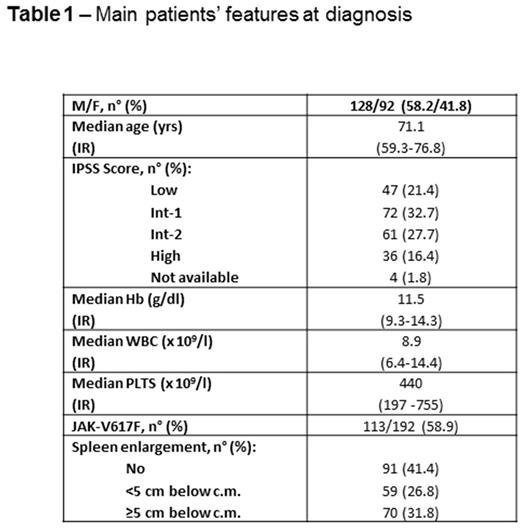
Methods. To address this issue, we report on 220 consecutive patients [M/F 128/92, median age 71.1 years, interquartile range (IR) 59.3-76.8] with newly diagnosed PMI according to the WHO 2008 criteria enrolled in the prospective database of our regional cooperative study group from January 2011 to December 2015. The main clinical features at diagnosis of the whole cohort are reported in the Table. Adverse events were considered thrombotic complications, evolution into blastic phase (BP) and deaths related or unrelated to PMI.
Results. On the whole, 10 episodes of early thrombotic complications were reported in 220 patients (4.5%) at a median interval from diagnosis of 33.0 months (IR 18.0-43.4): the 4-year cumulative thrombosis-free survival (TFS) of the whole cohort was 90.8% (95% CI 84.9-96.7). With regard to leukemic evolution, 18 patients (8.2%) evolved into BP after a median time from diagnosis of 23.9 months (IR 8.6-35.1): the 4-year cumulative leukemia-free survival (TFS) of the whole cohort was 87.2% (95%CI 81.3-93.1). Forty-five patients (20.4%) died within the first 4 years after diagnosis. The most common causes of death were evolution into BP (15 patients), evolution into an advanced PMI phase (10 patients), cardiologic complications (6 patients), infections (3 patients), thrombotic events (2 patients) and hemorrhages (2 patients). The 4-year cumulative overall survival (OS) rate of the whole cohort was 72.3% (95%CI 64.7-79.9). Several clinical features at diagnosis (age, gender, Hb levels, WBC and PLT counts, spleen enlargement, JAK-2 V617F mutation, IPSS score and previous thrombotic events) were evaluated for a role in predicting early adverse events. At univariate analysis, only Hb <10 g/dl (p=0.005) impacted significantly on a worse TFS and a IPSS Int-2/High (p=0.003) for a worse LFS. With regard to OS, several features were predictive of a worse prognosis at univariate analysis: male gender (p=0.007), IPSS Int-2/High (p<0.001), spleen enlargement absent or >5 cm (p=0.003), age >65 years (p<0.001), Hb <10 g/dl (p<0.001), WBC >15 x 109/l(p=0.004) and PLTs <700 x 109/l (p=0.017). In a multivariate Cox model, however, only IPSS Int-2/High retained an independent prognostic role for a worse OS (OR 10.4, CI 95% 0.042-0.460, p<0.001).
Conclusions. The incidence of adverse events within the first 4 years after diagnosis of PMF is relatively high based on our prospective database, with about one third of newly diagnosed patients affected. It is worth noting that factors classically involved in the thrombotic risk of other MPN, such as age and previous thrombotic events, did not seem to have a predictive role in PMF patients. On the other hand, our data strengthen the role of the IPSS score for LFS and OS prognostication
Breccia: Novartis: Consultancy; Pfizer: Consultancy; Incyte: Consultancy; Bristol Myers Squibb: Consultancy. Abruzzese: Pfizer: Consultancy; Novartis: Consultancy; BMS: Consultancy; Incyte: Consultancy. Foa: Janssen: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Sandoz: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.